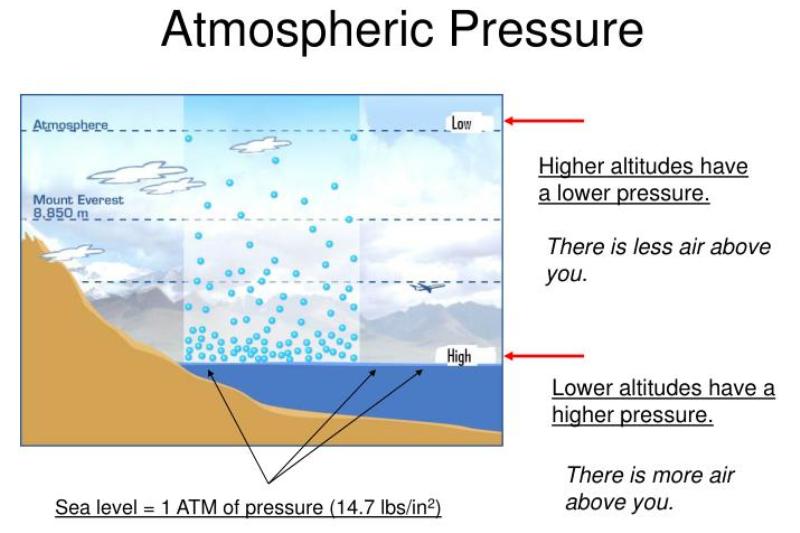
Second it reduces the weight of the air over the surface of the liquid which must be compensated for by increasing the concentration of. As the boiling point of a liquid is related to pressure putting the liquid in a vacuum will cause it to boil without applying heat.
Boiling Point Examples In Everyday Life Studiousguy
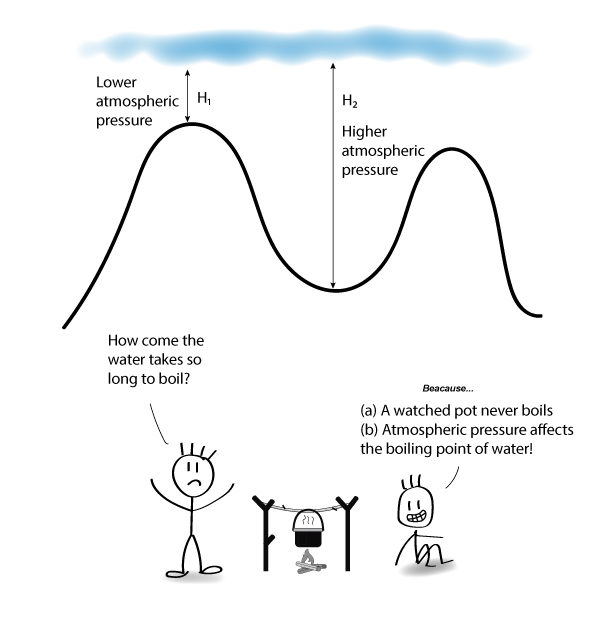
Why does lowering the pressure cause a liquid to boil.

. As the boiling point of a liquid is related to pressure putting the liquid in a vacuum will cause it to boil without applying heat. First it reduces the pressure of the atmosphere above the liquid which must be compensated for by raising the temperature of the liquid or its vapor will boil at some lower pressure. Elevation has two important effects on boiling points.
It causes the temperature to rise. It pushes molecules out of the liquid. It becomes easier for molecules to escape.
So when the liquid boils it no longer only loses molecules through its top interface but it can lose molecules into. It changes the volume of the liquid. As soon as the vapor pressure of the liquid becomes higher than ambient pressure bubbles of pure vapor can occur under the liquid surface.
Boiling point of a substance is the temperature at which the vapour pressure of the liquid equals the pressure surrounding the liquid and the liquid changes into a vapor spontaneously Hence if air pressure is low the vapour pressure has to achieve less pressure against boiling and as a result less temperature is needed to be provided to reach boiling point.
How Does Atmospheric Pressure Affect Boiling Point

0 Comments